Jg Oc
500 Determine the change in enthalpy for this reaction (you may Google the heats of formation necessary) H 2 S(g) O 2 (g).

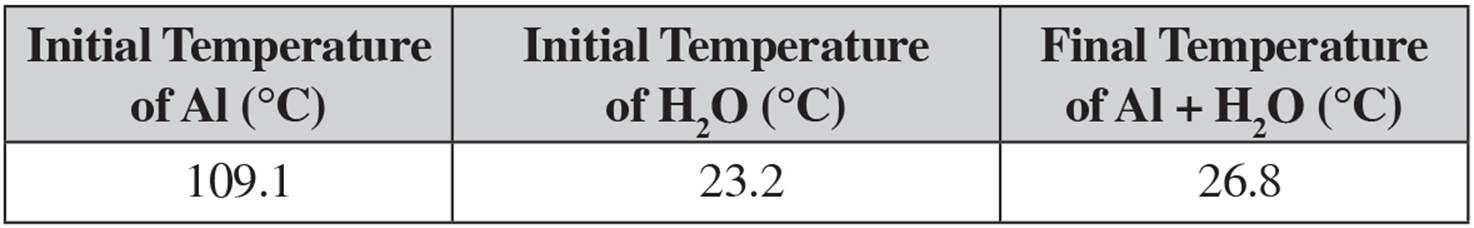
Jg oc. Qwater= J Cmetal= 0402 J/g oC 6 In a coffeecup calorimeter, 1000 g of H 2 O and 1000 mL of HCl are mixed The HCl had an initial temperature of 446 oC and the water was originally at 246 oC After the reaction, the temperature of both substances is 313 oC aWas the reaction exothermic or endothermic?. Share your videos with friends, family, and the world. 5 An aluminum can with a mass of 125 grams (Cp = 90 J/g o C) absorbs 245 Joules of heat How much does the temperature rise?.
Jun 21, 19 · 👍 Correct answer to the question Gold has a specific heat of 0129 J/g °C How many joules of heat energy are required to raise the temperature of 23 grams of gold from 22 °C to 98 °C?. 1) The specific heat of aluminum is 0900 J/g oC How much heat is required to raise the temperature of a 300g block of aluminum from 250oC to 750oC?. What is 655 g?.
The specific heat of lead is 0129 J/g°C, and the specific heat of water is 418 J/g°C When the temperature stabilized, the temperature of the mixture was 179°C Assuming no heat was lost to the surroundings, what was the mass of lead added?. AP® CHEMISTRY 10 SCORING GUIDELINES Question 2 (continued) (c) Assume that the specific heat capacity of the calorimeter is negligible and that the specific heat capacity of the solution of urea and water is 42 J g −1 °C 1 throughout the experiment (i) Calculate the heat of dissolution of the urea in joules. The heat of fusion of water is 602 kJ/mol, and the molar heat capacity is 366 J/mol K for ice and 753 J/mol K for liquid water Solution.
A J » (G• `A) GP O A) J (AG) 2, Com Ob) (J G) • A 2, Assoc O C) P 1, 3, MP Od J 1, 2, MP Given The Following Premises 1 (K•~C) V (K• ~H) 2 ~D = (K ~H) 3 ~(K• ~H) A) ~KVH 3, DM B) K• (~CV ~H) 1, Dist O C) C) Ko~C 1, 3, DS D) D 2, 3, MT Uction Point) Given The Following Premises 1. C for water = 418 J/g oC 2 How much heat in kilojoules has to be removed from 225g of. J&G TRADE SP Z O O is located in Piaseczno, MAZOWIECKIE, Poland and is part of the Beer, Wine & Distilled Spirits Wholesalers Industry J&G TRADE SP Z O O has 1 employees at this location and generates $79,173 in sales (USD).
A 45g aluminum spoon (specific heat 0 J/g °C) at 24 °C is placed in 180 mL (180 g) of coffee at 85 °C and the temperature of the two become equal What is the final temperature when the two become equal?. The specific heat capacity of porcelain is around 08 J/g·°С A typical porcelain espresso cup weighs about 60 grams, so it has a heat capacity of about 50 J/°С The specific heat capacity of water is 42 J/g·°С Thus, the heat capacity of a 50 g espresso shot is about 210 J/°С. Look at picture ASAP please will mark as brainlist don’t eeduanswerscom.
Enthalpy of Diluting a Strong Acid Theadditionofastrongacidtowatergeneratesheat;thatis,thereactionisexothermic. Q = (150 g) ( J g¯ 1) = J Note that the 100 g of water is not mentioned yet 2) Determine heat need to raise 115 g of water from 0 to 100 °C q = (115 g) (100 °C) (4184 J g¯ 1 °C¯ 1) = J Note the inclusion of the melted 15 g of ice Also, notice that the water was at zero °C We know this from the presence of. Assume that coffee has the same specific heat as water The first time a student solved this problem she got an answer of °C.
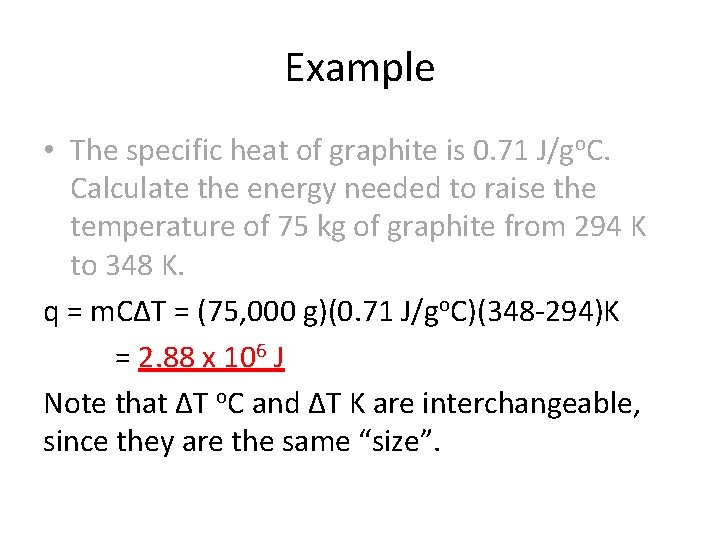
Jan , 18 · I'm confused because the answer uses specific heat capacity of liquid H 2 0 which is 4184 J/g C to find the heat of the ice cube The heat absorbed by the ice cube involves the heat absorbed by melting the ice at 0 o C to liquid water at 0 o C, and the heat absorbed by raising the same amount of liquid water at 0 o C to liquid water at the. 1 The temperature of 335 g of water changed from 245oC to 264oC How much heat did this sample absorb?. J/g×°C or J/g×K since 1oC = 1 degree K m = mass, measured in grams Δt = temperature change, °C or K NOTE All temperatures in this lab will be recorded in degrees Celcius, oC 2 Part I Heat Capacity of the Calorimeter.
The specific heat of water is 4184 J/g o C Assume that these solutions are close enough to being like water that their specific heats are also J/g o C The density of water is 100 g/mL and even though these are solutions we can assume that they are close enough to. The specific heat capacity of solid aluminum (0904 J/g/°C) is different than the specific heat capacity of solid iron (0449 J/g/°C) This means that it would require more heat to increase the temperature of a given mass of aluminum by 1°C compared to the amount of heat required to increase the temperature of the same mass of iron by 1°C. Q = 250g x 418J/g o C x 26 o C q = 37,6J or 38kJ 2 Calculate the specific heat capacity of copper given that 475 J of energy raises the temperature of 15g of copper from 25 o to 60 o q = m x C x DT C= q/m x DT C = 475J /(15g x 35 o C ) C= 039 J/g o C.
Alorimet Problem qsur=m x C x AT q = heat m = mass Name Per Date qrxn q sur AT = TfTl C = specific heat (for water = 4184 J/g0C) 1 When a 257 g sample of Nal dissolves in 800 g of water in a calorimeter, the. 26 The temperature of a silvers coin (C= 024 J/goC) falls by 353 oC as it releases 5,550 Joules of heat What is the mass of the coin?. 25 A 500 g block of glass (C= 050 J/goC) absorbs 333 joules of heat energy How much does the temperature of the glass rise?.
Problem #4 A 500 g sample of aluminum (specific heat capacity = 0 J g¯ 1 °C¯ 1) and a 1000 g sample of iron (specific heat capacity = 045 J g¯ 1 °C¯ 1) are heated to 1000 °CThe mixture of hot iron and aluminum is then dropped into 919 g of water at 237 °C Calculate the final temperature of the metal and water mixture, assuming no heat loss to the surroundings. Of tin is J/goC 930 J 4 If it takes 24,500 J to heat 105 g of a substance from 25oC to 49oC, what is the specific heat of the substance?. Mass = (425 g x 0133 J/g deg x 754 o) / (418 J/g deg x 25 o) = 408 g mass = _41 g_____ 4 A chemical compound has a molecular weight of 05g/mole 1400 g of this compound underwent complete combustion under constant pressure in a calorimeter with a heat capacity of 2980 J o C1 The temperature went up by 1195 degrees Calculate.
No, gold had a specific heat capacity of 0129J/g o C and lead of 0128J/g o C The thousandths position is uncertain and so to three significant digits, you can not differentiate between these two samples (you report all certain and the first uncertain, so if you have a measurement of 0128, you are not really sure of the 0008 value. May 02, 21 · specific heat of steam = 9 J/g·°C Solving the Problem The total energy required is the sum of the energy to heat the 10 °C ice to 0 °C ice, melting the 0 °C ice into 0 °C water, heating the water to 100 °C, converting 100 °C water to 100 °C steam and heating the steam to 150 °C To get the final value, first calculate the. (e) If a sample of gas is heated from 473 oC to 1219 oC, the volume will increase by a factor of two TRUE 1492 K (from 1219 o C) is double of 746 K (from 473 o C).
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators. JGOC Business Supplier from Paraguay View Company Commodity Chia Seedstype Organic Chia Seeds Chia Is 100% OrganicPurity 99,8%Offer For Organic Chia Seeds (salvia Hispanica) Quantity Available 100,000 Bags Origin Kenya Specification Purity 99% Color Black/grey Colored Seeds Size 1721 Mm Foreign Matter 01% Damaged Seed 01% Moisture 10% Organic. Nov 09, 19 · Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquidIt's also known as enthalpy of fusion Its units are usually Joules per gram (J/g) or calories per gram (cal/g) This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice.
27 An aluminum can with a mass of 125 grams (C= 90 J/goC) absorbs 245 Joules. Figure 1 In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an endothermic process occurs and heat, q, is positive, indicating that thermal energy is transferred from the surroundings to the system. 970 J 5 If 24,500 J is applied to 125 g of water at 35oC, what will the final temperature of the water be?.
The specific heat of the liquid is 0 J/g C. D h a n r a j G o c h a r Media Send Message D h a n r a j G o c h a r February 28, 18 · Love youtubecom Love stetus Love Love D h a n r a j G o c h a r is feeling crazy November 12, 17 · D h a n r a j G o c h a r updated their cover photo November 11, 17 · D h a n r a j. A 0540 J (Incorrect) b 150 J (Incorrect) c 1350 J (Correct) d 1670 J (Incorrect) 2) Given the balanced equation representing a reaction at 1013 kPa and 298K N 2 (g) 3H 2 (g) 2NH 3.
4524 x 10 3 J g oC = oC = oC or 1355 x 10 2 oC 7421g • 045 J 6 What was the initial temperature of a 325 g iron bar if 2768 J of energy were applied resulting in a final temperature of 954 oC?. The temperature of a silver coin (C p = 0240 J/g o C) falls by 353 o C as it releases 5,550 Joules of heat What is the mass of the coin?. Specific heat ice = 3 J/ g o C specific heat water = 418 J/g.
The temperature of a silver coin (Cp = 024 J/g o C) falls by 353 o C as it releases 5,550 Joules of heat What is the mass of the coin?. The specific heat of ice is the heat that should be supplied to a unit mass of ice for a unit temperature rise We learn that heat worth {eq}\rm 334 \ J {/eq} has to be supplied to {eq}\rm 1 \. How much energy does a copper sample absorb as energy in the form of heat if its specific heat is 0384 J/(g·ºC), its mass is 800 g, and it is heated from 100ºC to 400ºC?.
A i B Z h i œ { l ̊ό c A Ă ܂ B p X b g Ƃ Ēm { e b N X ŃG l M 邱 Ƃ ł l C ̃Z h i B A i B Z h i ŃI v V i c A R p ӂ Ă ܂ BAntelope Canyon, Sedona, Grand Canyon, Monument Valley, Optional Tour. 9 If 5750 joules of energy are added to 455 grams of granite at 2400 C, what is the final temperature of the granite?. 55°C, if the specific heat of aluminum is 090 J/g°C?.
A) 157 kg B) 170 g C) 4 g D) 211 g. Cp granite is 079 J/g oC ΔT = Q = 5,750 J = 5,750 J x oC = 160 oC 455 g 079 J J J g oC oC. Example #5 How much energy in kJ is needed to heat 500 g of ice from −100 °C to 300 °C?.
Answer to 1How much heat, in kJ, is needed to warm 130 g of ice from 500 o C to 370 o C?. 4 Calculate the amount of energy (in kJ) needed to heat 346 g of liquid water from 0C to 1C Assume that the specific heat of water is 4184 J/g C over the entire liquid range and the specific heat of steam is 199 J/g C The molar heat of vaporization of water is 4079 kg/mol. Or Create New Account Not Now Pages Media D h a n r a j G o c h a r Videos English (US) Español;.
(The specific heats of ice, water, and. A metal has a specific heat of 0250 J/g o o o o C If 100 g of the metal at 0 C is added to 300 g of a liquid at 600 C in a calorimeter, what will be the final temperature to the nearest degree?. Answer to How much heat (in kJ) is needed to convert 866 g of ice at 100^{o} C to steam at 1260^{o} C?.
Assume the specific heat of copper to be 0385 J/g°C and determine the amount of heat absorbed by the block of copper Answer ΔH = C p ΔT = (850 g)(0385 J/g°C)(7 °C) = 2291 J = 23 kJ;. The specific heat capacity of solid iron is 045. See more of D h a n r a j G o c h a r on Facebook Log In or Create New Account See more of D h a n r a j G o c h a r on Facebook Log In Forgot account?.
May , 18 · 254 J/mol The specific heat of any substance is referring to the amount of energy require to raise 1 gram of that substance 1 degree kelvin Molar heat capacity is referring to the amount of energy required to raise 1 mole of that substance 1 degree kelvin So in order to solve for molar heat capacity of gold we must convert from grams of gold to moles of gold. OC 6 A reactor core needs to stay at or below 95oC to remain in good condition Cool. A 0258 g piece of potassium solid is placed into water inside of a coffee cup calorimeter resulting in a vigourous reaction Assume a total volume of.
016 g 62 g 45 x 10 3 g x 10 4 g None of these are correct A 630 g piece of aluminum (Specific Heat = 0215 cal / (g o C) ) at 250 o C is warmed by the addition of 325 calories of energy Find the final. Feb 28, 15 · In order to answer this question, you you will need to use the following equation #q = cmDeltaT#, where #q# is the quantity of heat gained or lost, #c# is the specific heat capacity (of water in this case), #m# is mass in grams, and #DeltaT# is the difference in temperature, #DeltaT=T_"final"T_"initial"# Known/Given #c_"water"= 4184 "J"/("g"*""^("o")"C"#. 350 J are released as ice ( Specific Heat = 21 J / (g o C) ) cools from 50 o C to 32 o C What is the mass of ice?.
Thermodynamics Energy Is The Ability To Do Work

Stc Go 22x Gl225 Technical Data Sheet Manualzz

This Third Party Surface Connect To Usb C Adapter Is A Must Have For Surface Users Onmsft Com
Jg Oc のギャラリー

Page 495 Problems 4 6 Page 495 Questions Ppt Download

Stranger Things Season 3 Lp
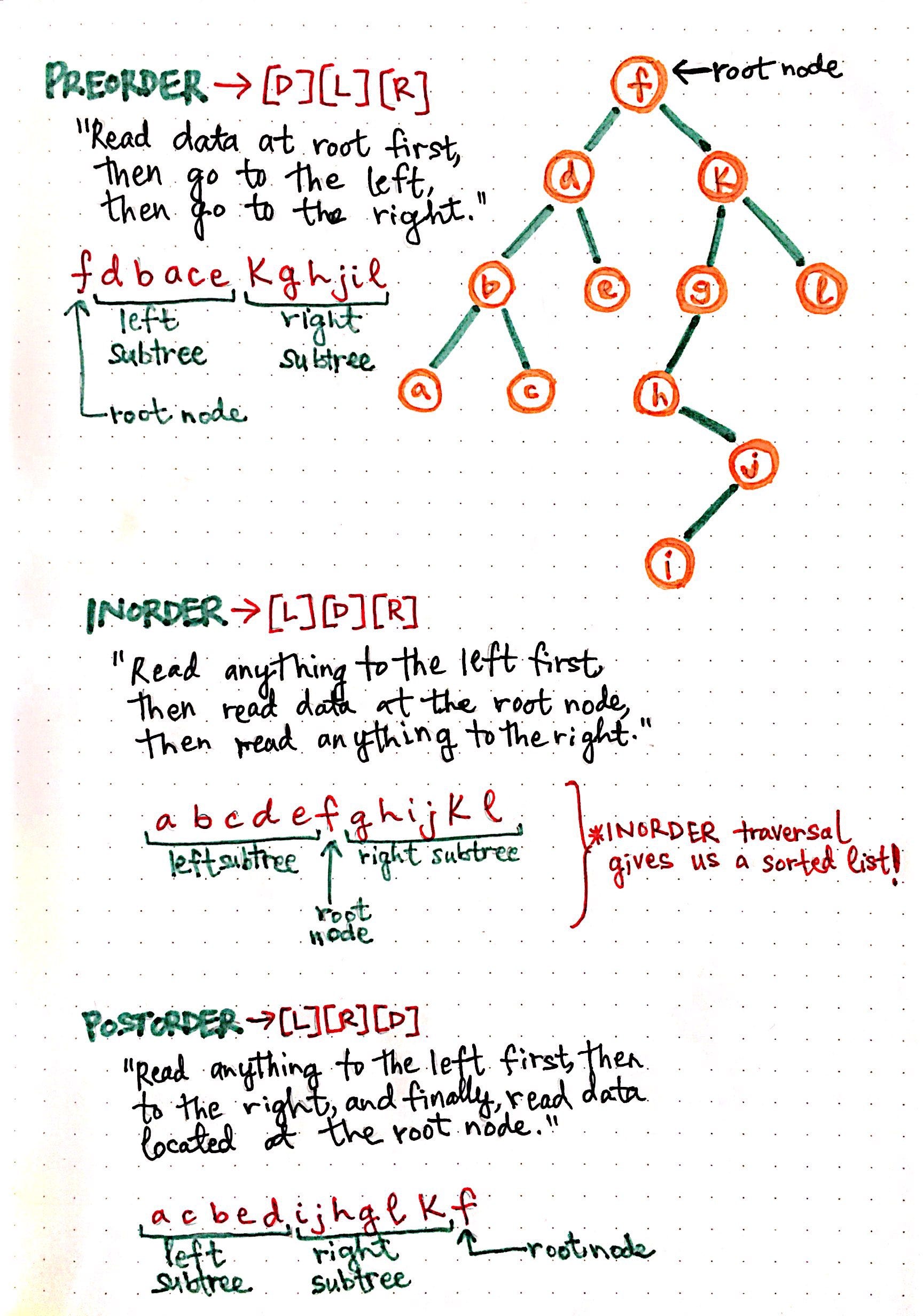
Demystifying Depth First Search Once You Ve Learned Enough About By Vaidehi Joshi Basecs Medium
:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/16126121/akrales_190416_3376_0007.jpg)
High Wattage Usb C Batteries Can Keep Your Laptop Charged On The Go The Verge

Explode The Code Go For The Code C School Specialty Eps

Given The Following Thermochemical Equations N 2 G O 2 G 2nog U0394h 1807 Kj 2nog O Course Hero

What Is The Change In Entropy When 2 5 Mole Of Water Is Heated Fro

C H Activation In Organic Synthesis European Journal Of Organic Chemistry Vol 18 No 44 Chemistry Europe

Global Malicious Spam Campaign Using Black Lives Matter As A Lure Fortiguard Labs
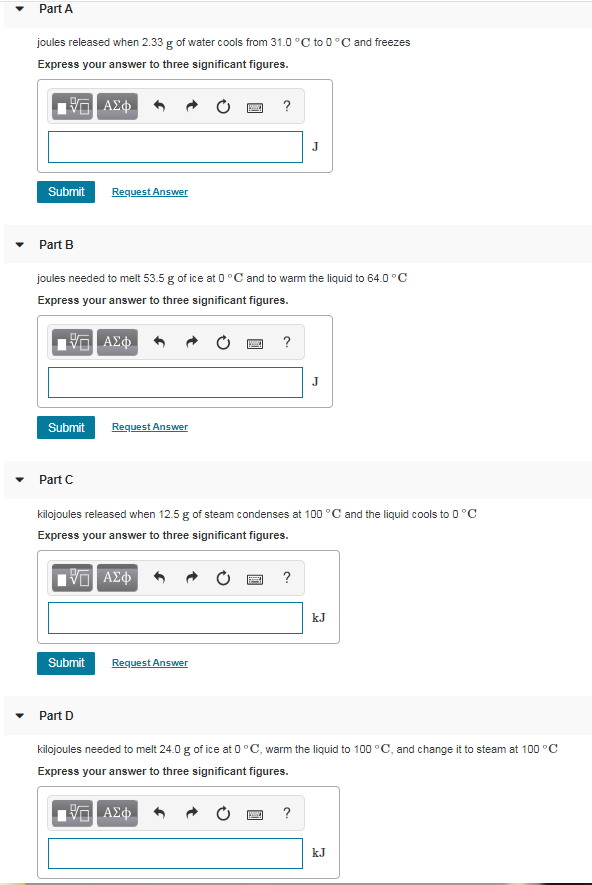
Solved Using The Heat Of Fusion For Water 334 J Gj G Th Chegg Com

Ch17 Thermo Review Answers
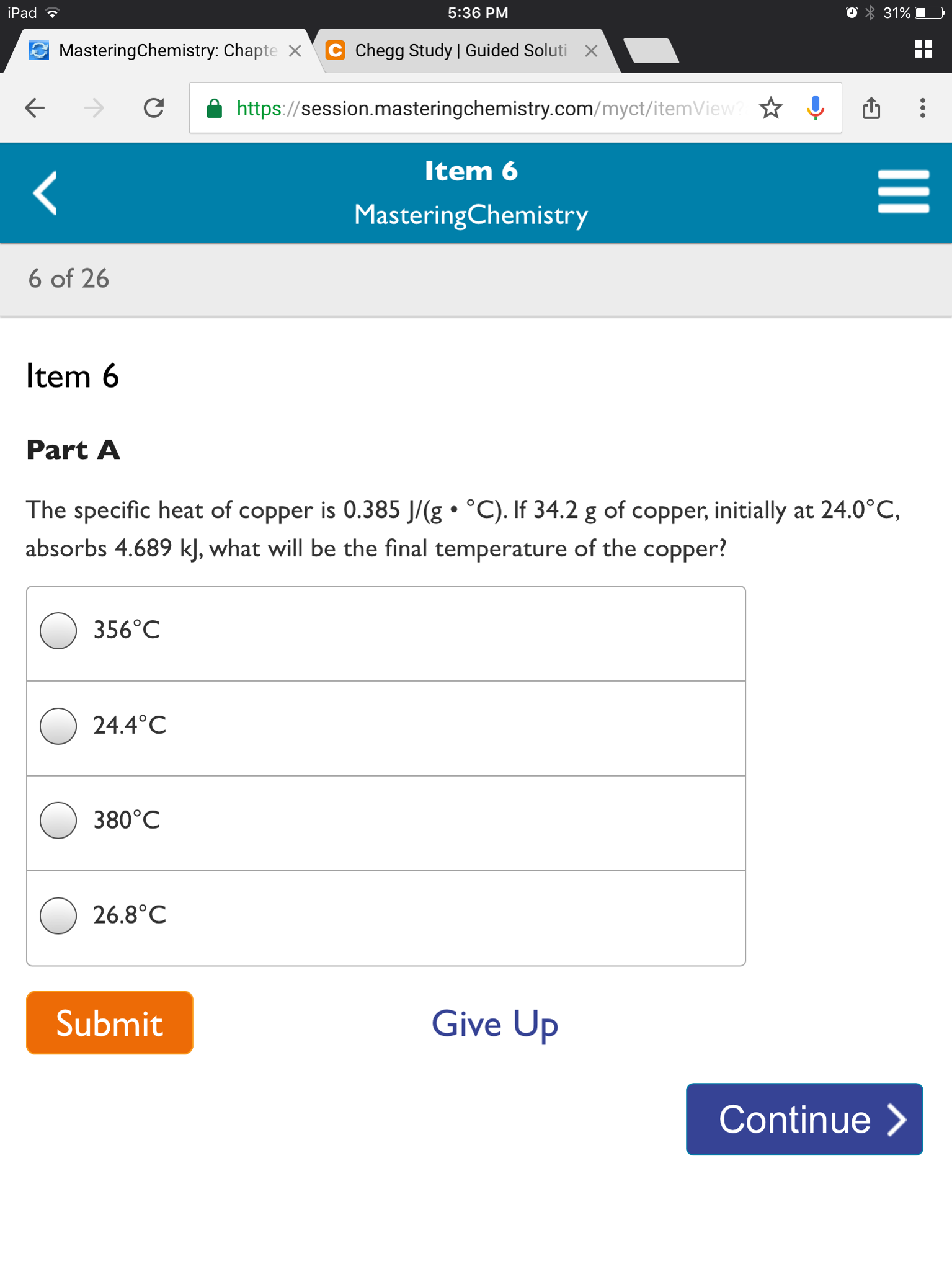
Solved The Specific Heat Of Copper Is 0 385 J G Middot D Chegg Com

Build A Command Line App With Go Lolcat

Thermal Behavior Of Graphene Oxide Coated Piassava Fiber And Their Epoxy Composites Sciencedirect

How To Go From 0 To 5m In Saas Revenue Within A Span Of 3 Months Without Any Vc Funding

Improvement Of Stability And Release Of Epicatechin By Hot Melt Extrusion

A 70 0 G Piece Of Metal At 80 0 C Is Place Clutch Prep
Chapter 7 Questions Laws Of Thermodynamics And Changes In Matter Content Review For The Ap Chemistry Exam Cracking The Ap Chemistry Exam

Thermochemistry Worksheet

Activity14calorimetryss Pdf Names Dr Guldan Chem 122 Activity 14 Using Calorimetry Solutions 1 As A Purity Check For Industrial Diamonds A 2 064 G Course Hero
Chord House Of Cards Owen Temple Tab Song Lyric Sheet Guitar Ukulele Chords Vip

Calculate The Amount Of Heat Required To Raise The Temperature Of 5 G Of Iron From 25 C To Youtube
As C E O I Try To Look At The Big Picture While J G As President Se New Yorker Cartoon Premium Giclee Print Charles Barsotti Art Com
Gary O Donoghue High Resolution Stock Photography And Images Alamy

Beet J Stag Rangerwiki Fandom
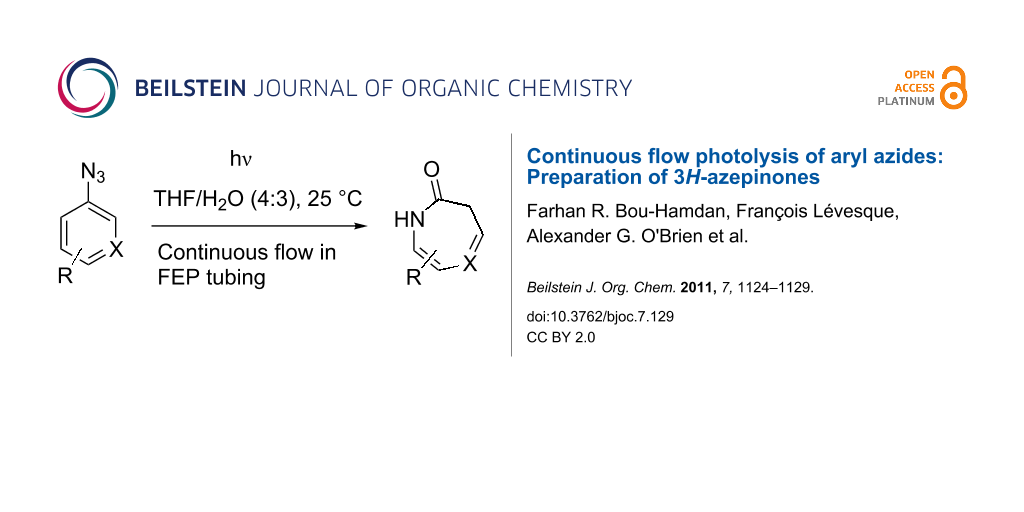
Bjoc Continuous Flow Photolysis Of Aryl Azides Preparation Of 3h Azepinones

A Hot Lump Of 106 2 G Of An Unknown Substa Clutch Prep

Ppt Energy And Chemical Change Powerpoint Presentation Free Download Id
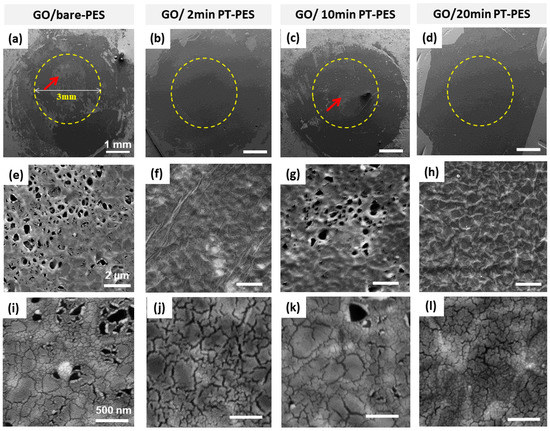
Membranes Free Full Text Graphene Oxide Based Membranes For Water Purification Applications Effect Of Plasma Treatment On The Adhesion And Stability Of The Synthesized Membranes Html

Illinois Pres Free Jg Tc Ad From 21 04 22 Office Services Jg Tc Com

J G Monnet Vsop Bot 1970s The Whisky Exchange

Microsoft Surface Connect To Usb C Charging Adapter 15v J Go Tech

Amazon Com J Go Tech Original Surface Connect To Usb C Charging Cable 15v 3a With Ce And Rohs Safety Certificates For Microsoft Surface Pro 3 4 5 6 7 Surface Book 1 Surface Go Surface Laptop 1 2 3 Electronics

Solved The Units For Heat Capacity Are Select One A J G Chegg Com

Z6f Oq6ognqs2m

Ppt Energy And Chemical Change Powerpoint Presentation Free Download Id

Tem Images Of Go And Rgo A Go B J Rgo C A Rgo And D N Rgo Download Scientific Diagram

August 15 1994 High Resolution Stock Photography And Images Alamy

10 Heat Of Fusion And Vaporization Worksheet 2

Surface Connect To Usb C Charging Cable By J Go Tech Requires Pd 45w 15v 3a Usbc For Sale Online Ebay
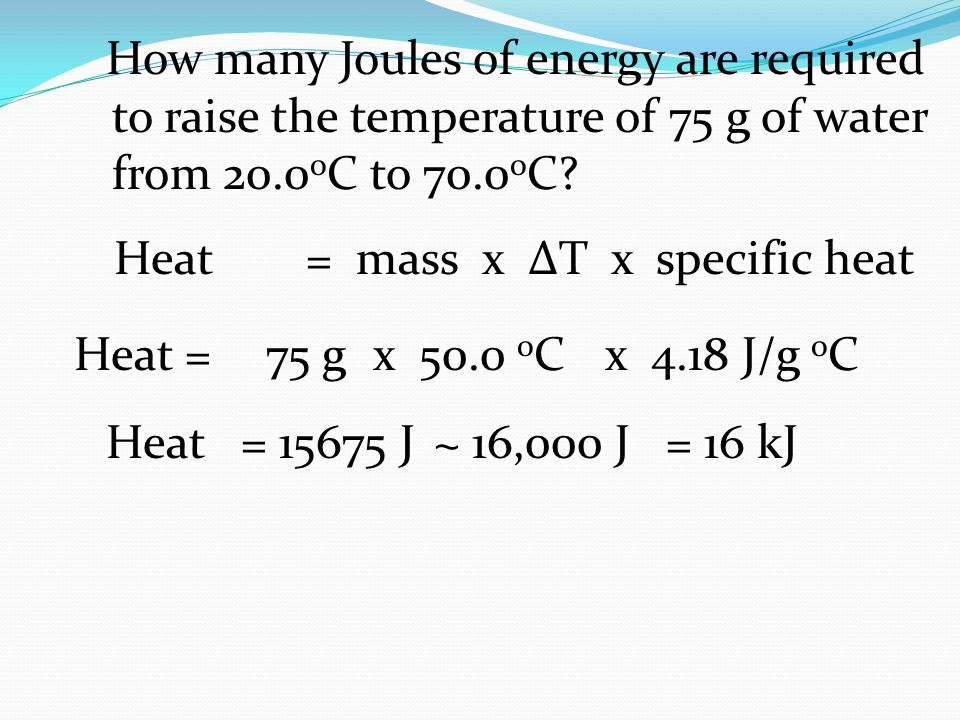
How Many Joules Of Energy Are Required To Raise The Temperature Of 75 G Of Water From 0 O C To 70 0 O C Heat 75 G X 50 0 O C X

Specific Heat Calculator
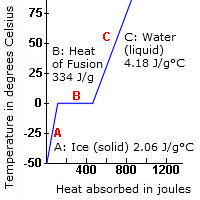
Arctic News Latent Heat

J Go Tech The Tanker Review Switch Chargers

High Capacity 45w Power Bank Tsa Approved Power Bank By J Go Tech

Analysis Of Oxidation Degree Of Graphite Oxide And Chemical Structure Of Corresponding Reduced Graphite Oxide By Selecting Different Sized Original Gr Rsc Advances Rsc Publishing Doi 10 1039 C8rah

Microsoft Surface Connect To Usb C Charging Adapter 15v J Go Tech

During An Experiment A Student Adds 1 23 Clutch Prep
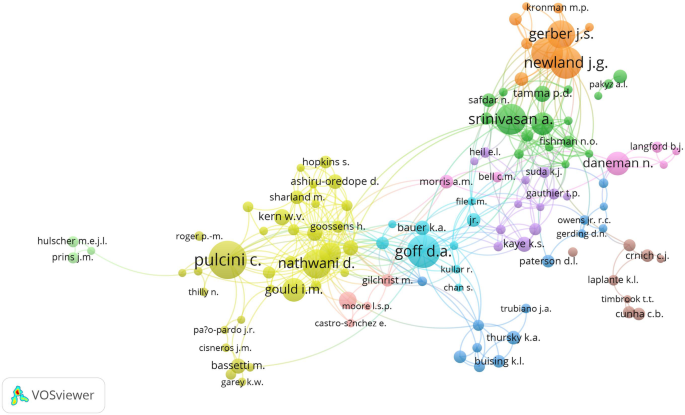
Bibliometric Analysis Of Peer Reviewed Literature On Antimicrobial Stewardship From 1990 To 19 Globalization And Health Full Text

Worship 2 Go Generosity All Saints C Of E Primary School

Formation Of Composite Polyaniline And Graphene Oxide By Physical Mixture Method

Chord American Jesus Bad Religion Tab Song Lyric Sheet Guitar Ukulele Chords Vip

Amazon Com The Tanker Pd 45w Surface Portable Charger By J Go Tech mah Portable Charger Usb C Input Output Power Delivery Charging For Usb C Devices Electronics

Dr Seuss Week Bulletin Board Book Oh The Places You Ll Go By Kl Jg Dr Seuss Week Dr Seuss Bulletin Board Dr Seuss Classroom
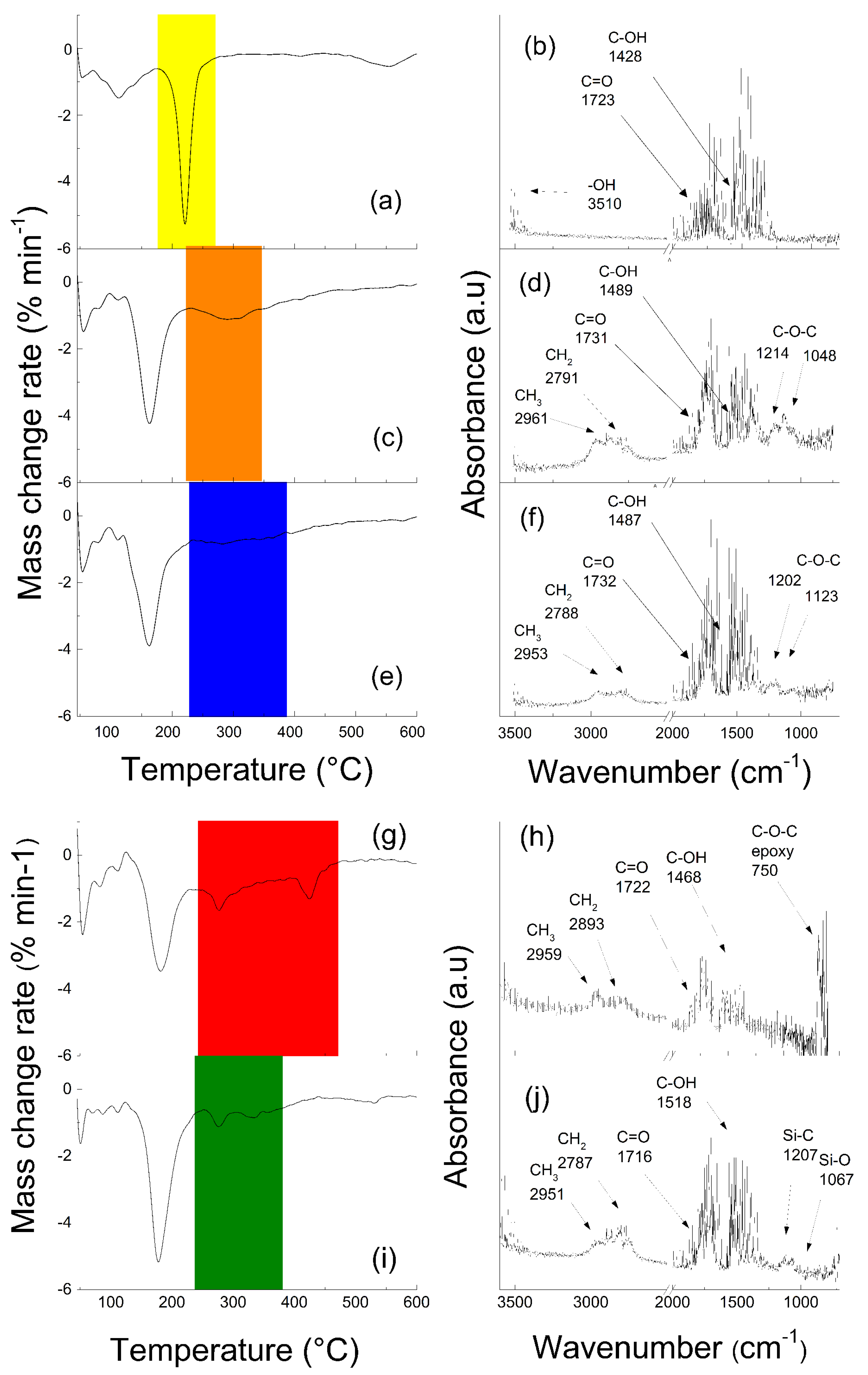
Nanomaterials Free Full Text Effect Of Structure Of Polymers Grafted From Graphene Oxide On The Compatibility Of Particles With A Silicone Based Environment And The Stimuli Responsive Capabilities Of Their Composites Html

The Chinamen Must Go Library Of Congress

Mercury Metal Has A Specific Heat Capacity Of 0 140 J G Degrees C How Many In Joules Would It Take To Raise The Temperature Of An 11 0 Gram Mass Of Mercury From 0 Degrees C To 30 0 Degrees C

Tacita Dean Exhibitions Frith Street Gallery

Chem 180 Set 1 Flashcards Quizlet

Chemistry Chemical Equations Pdf Free Download

C V Plots Of The Mimcaps A Untreated B 5 Min And C 10 Min H 2 O Download Scientific Diagram
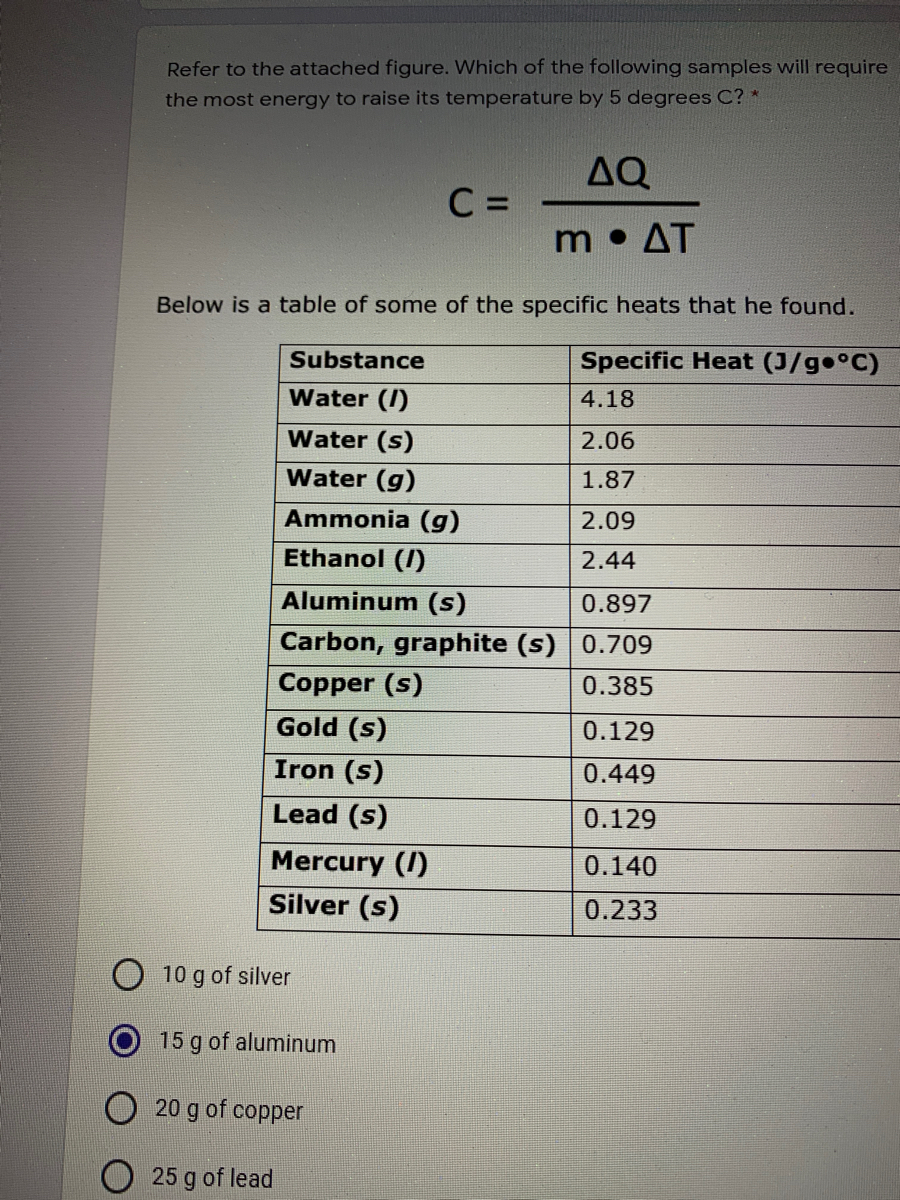
Answered Refer To The Attached Figure Which Of Bartleby

Narrative Economics Princeton University Press

Epb2 A Thermal Control Device Google Patents

How Much Energy Is Needed To Convert 23 0 Grams Of Ice At 10 0 C Into Steam At 109 C Socratic

Which One Of The Following Would Raise The Temperature Of G Of

Chemistry 5 2 Flashcards Quizlet

Answered 5 75 0 Ml Of Distilled Water At 28 00 Bartleby
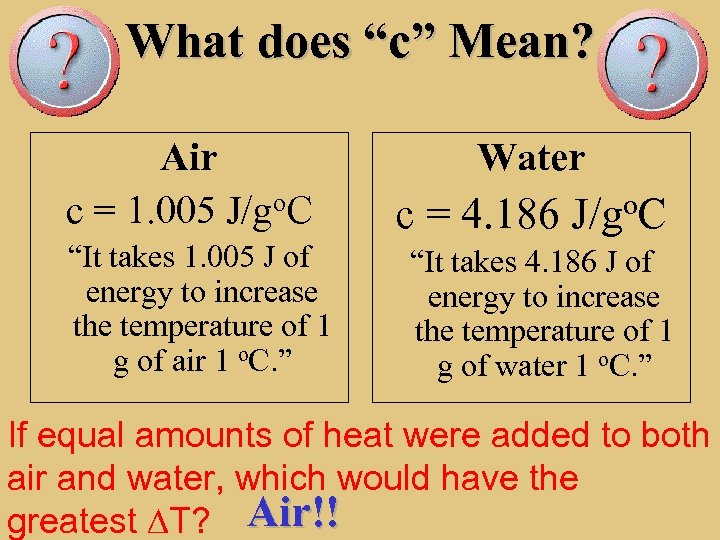
Def A Measure Of Average Kinetic Energy
After Transfer Of 1 50 Kj Of Thermal Energy To A 0 663 Kg Block Of Copper The Temperature Is 47 8 C Brainly Com

Jgo Cbd Gummies 1000 Mg Strength Assorted Flavor Party Pack Cbdpenshop Com

How Many Joules Of Energy Are Required To Raise The Temperature Of 75 G Of Water From 0 O C To 70 0 O C Heat 75 G X 50 0 O C X
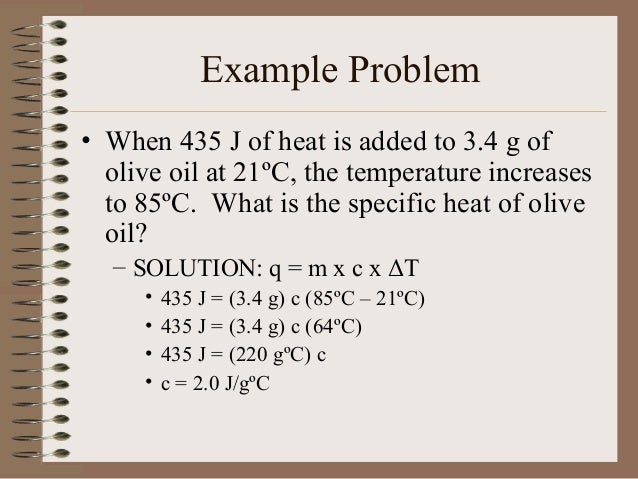
Thermochemistry

Heat Worksheet Specific Heat And Heat Capacity Worksheet 1 The Temperature Of 335 G Of Water Changed From 24 5oc To 26 4oc How Much Heat Did This Course Hero

Surface Connect To Usb C Charging Cable Pd 15v By J Go Tech

Comparison Of Fecal Microbiota Composition Of Blue Sheep Fed Lolium Perenne Versus Sorghum Sudanense

As C E O I Try To Look At The Big Picture While J G As President Se New Yorker Cartoon Premium Giclee Print Charles Barsotti Allposters Com

J Go Tech Original Surface Connect To Usb C Charging Cable 15v 3a With Ce And Rohs Safety Certificates For Microsoft Surface Pro 3 4 5 6 7 Surface Book 1 Surface Go Surface Laptop 1 2 3 Walmart Com
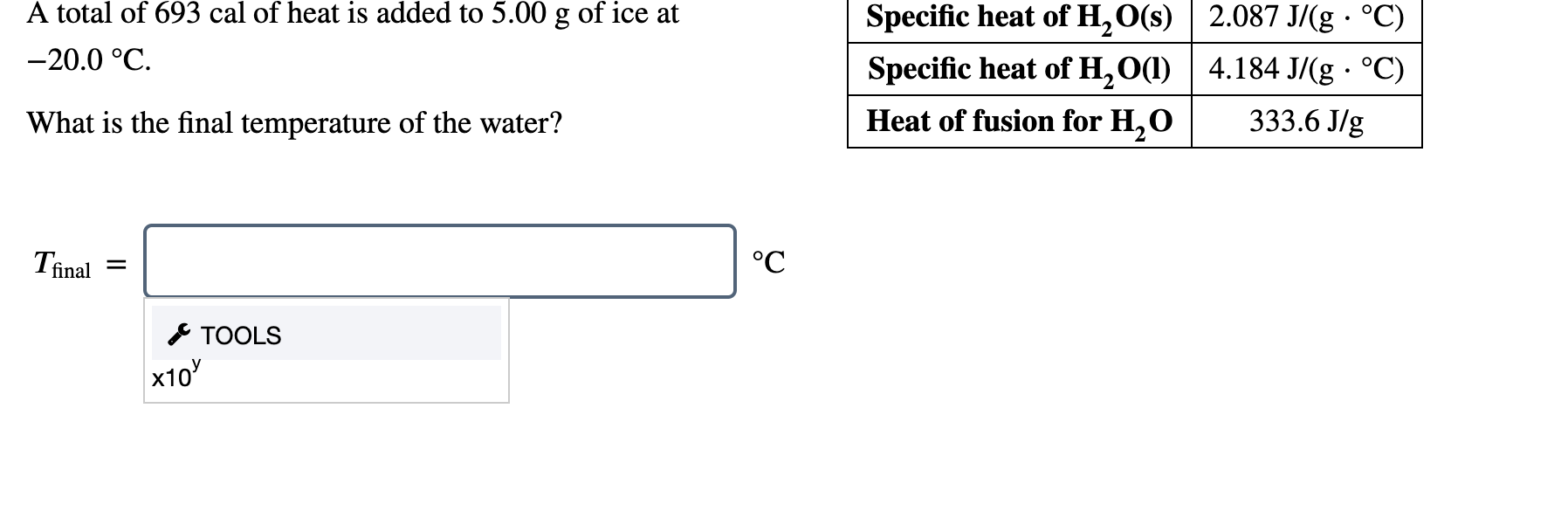
Solved A Total Of 693 Cal Of Heat Is Added To 5 00 G Of I Chegg Com
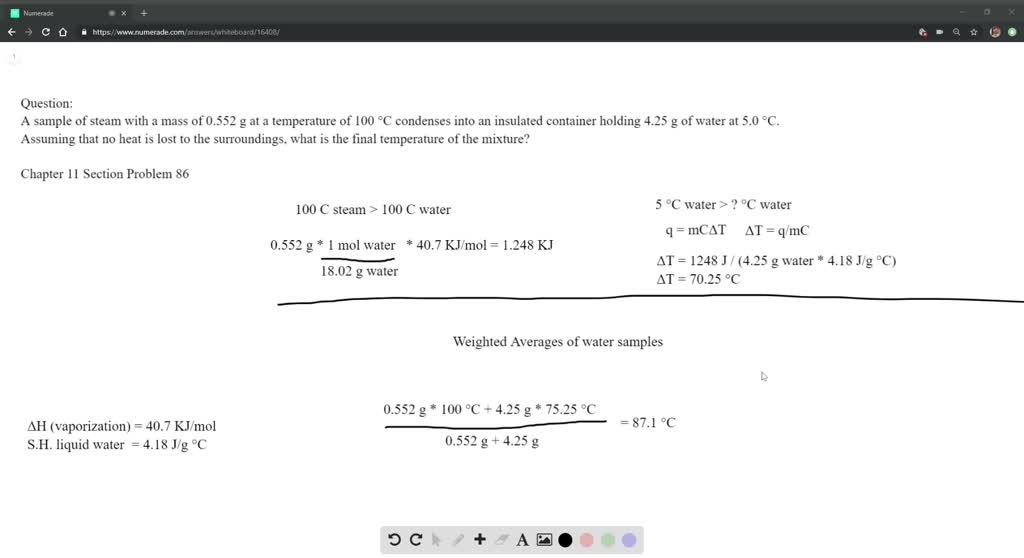
Solved A Sample Of Steam With A Mass Of 0 552 G A

Oneclass Specific Heat Capacity Of Water 4 184j G C Specific Heat Capacity Of Ice 2 06 Jg Heat O
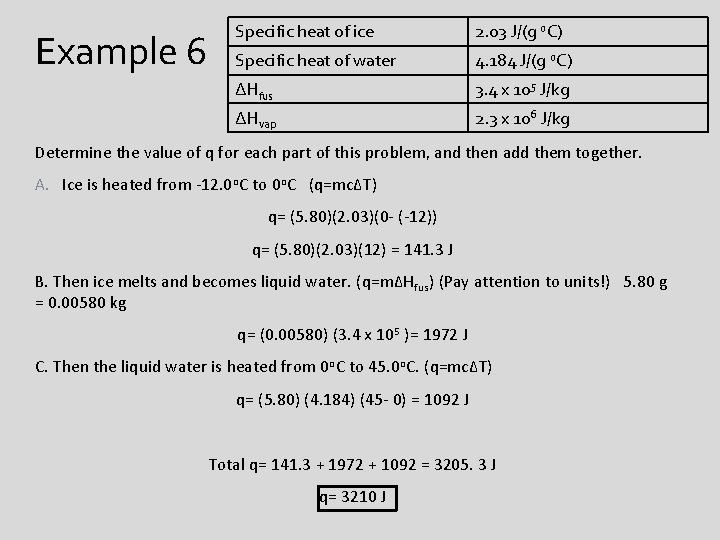
Unit 12 Energy And Thermodynamics What Is Energy

National Holidays Brand Acquired By Jg Travel Group Travel Weekly
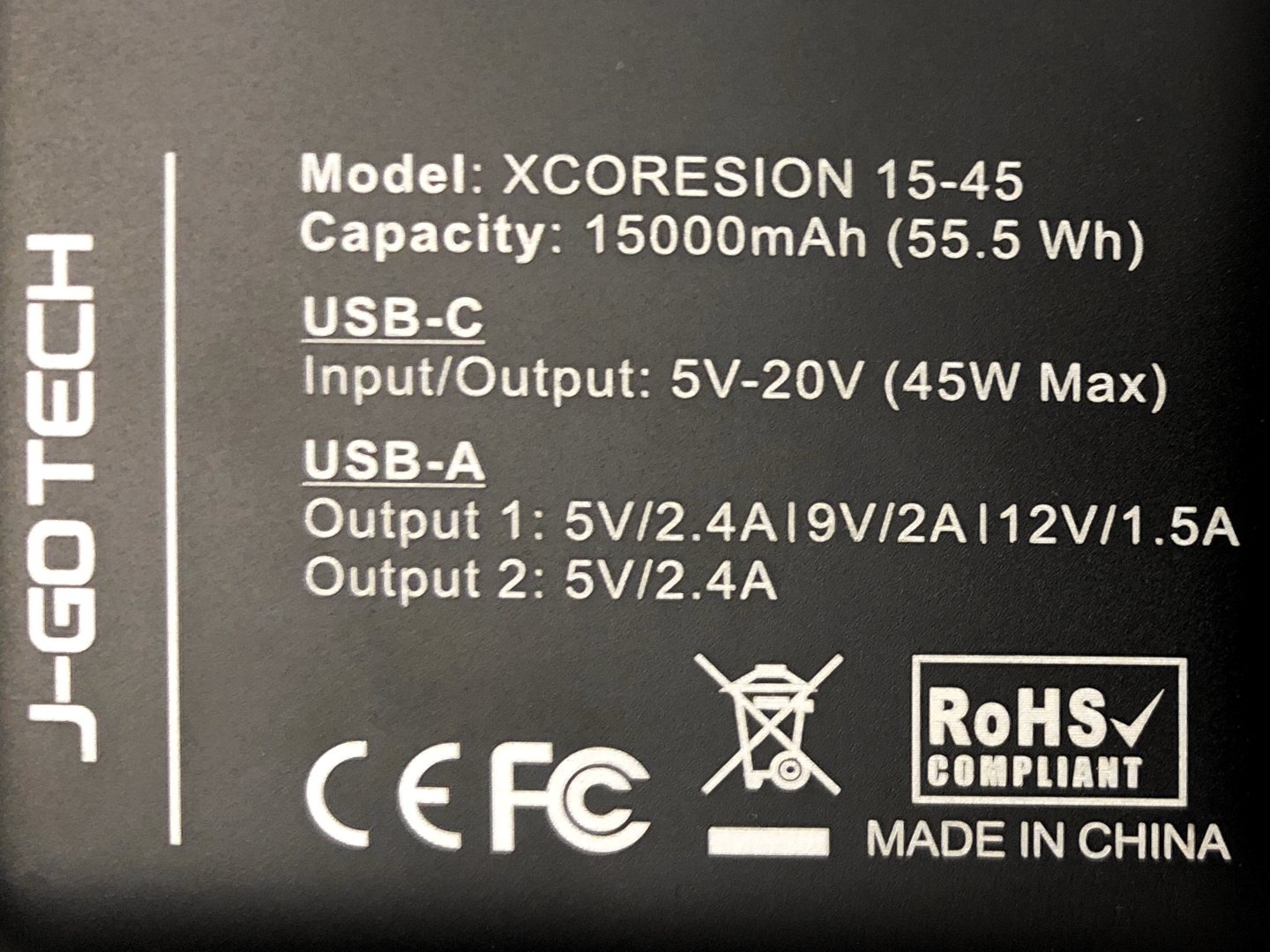
J Go Tech Xcoresion 15 45 Review Switch Chargers

Homework Assignment 1 Chapters 2 4 Flashcards Quizlet
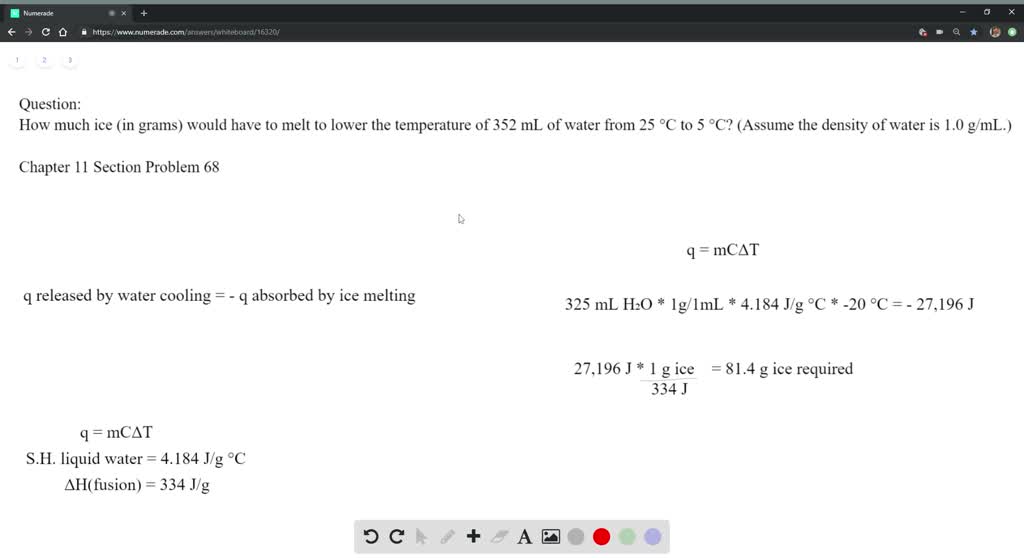
Solved How Much Ice In Grams Would Have To Melt

1 Vytah
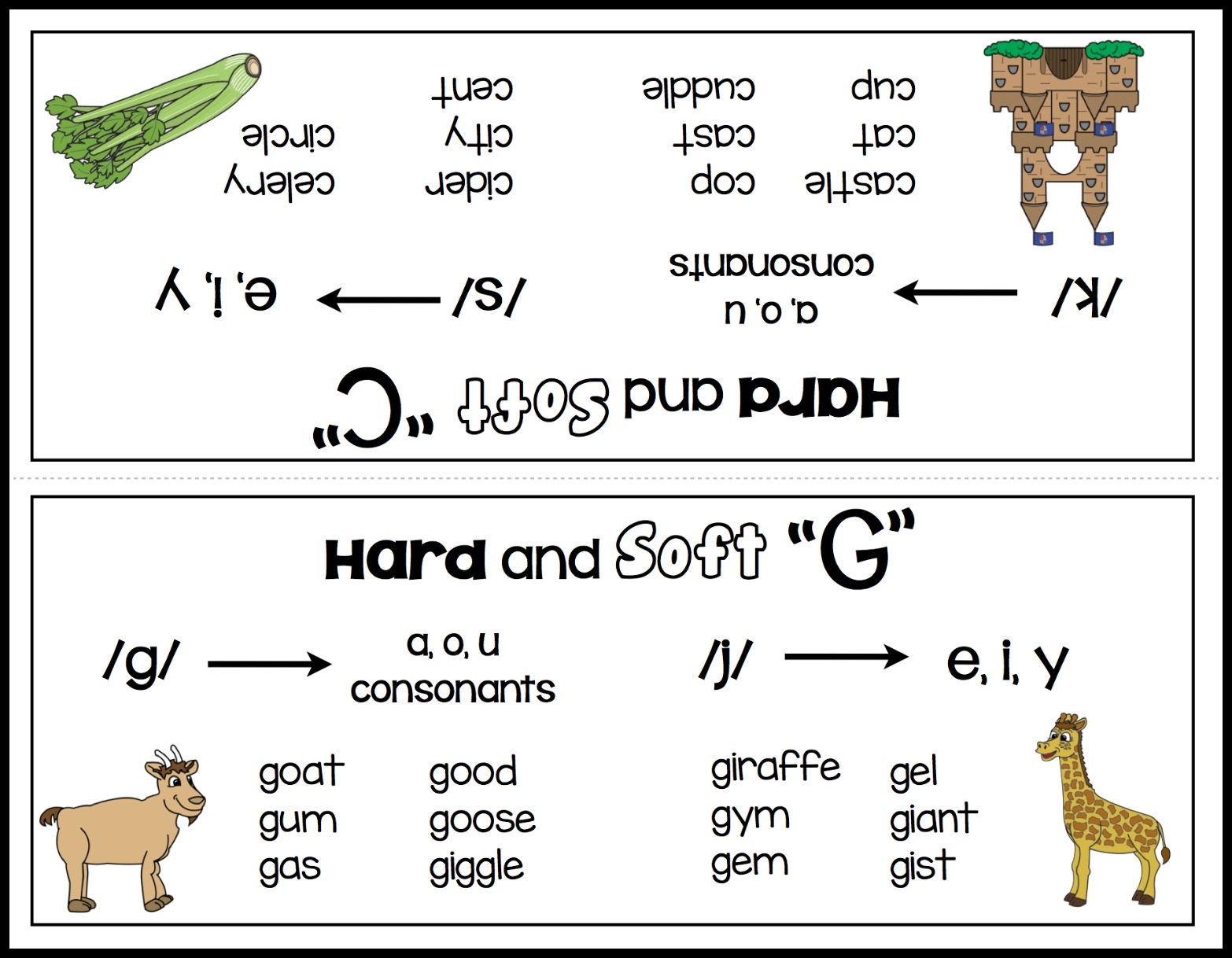
Teaching The Hard And Soft C And G Make Take Teach

Unit 12 Energy And Thermodynamics What Is Energy




